Abstract
Background: Follicular lymphoma (FL) is a common, indolent, yet typically incurable disease characterized by clinical and genetic heterogeneity. Recent studies suggested that TNFRSF14 mutation and 1p36 deletion were associated with worse outcome in FL and that TNFAIP3 mutation on 6q occurred at FL transformation. However, no genomic biomarkers are currently in clinical use for prognostic and therapeutic decisions. We aimed to identify novel genomic aberrations associated with prognosis of patients with FL in the context of a randomized cooperative group trial (Press 2013 JCO).
Patients and Methods: We employed a comprehensive genomic array testing strategy to assess the common genomic abnormalities, including copy number aberrations (CNAs), copy-neutral loss-of-heterozygosity (cnLOH), and common cancer gene mutations. Our study set includes all patients enrolled in the SWOG FL study S0016 with available pre-treatment tissue specimens. Chromosome genomic array testing (CGAT) has been performed on 255 archived formalin-fixed, paraffin embedded (FFPE) lymphoma tissue specimens. A SWOG pathologist reviewed all tissues prior to CGAT to ensure that each sample met the diagnostic criteria for FL and had at least 30% tumor content. The OncoScan platform was used for CGAT with data analysis performed by Nexus Express. Statistical analysis was performed using the Cox-proportional hazard regression model. Hazard ratio (HR) and 95% confidence interval (CI) were estimated from the multivariate model. Statistical significance was determined at an alpha-level of 0.05 after Bonferroni adjustment for multiple comparisons.
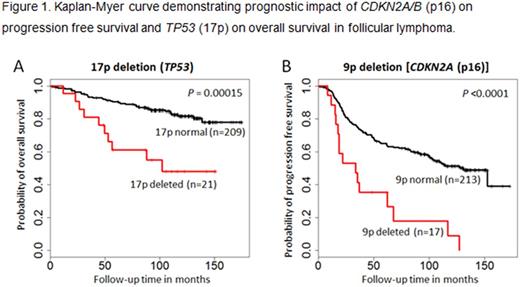
Results: CGAT was successful on 247 samples (97%), and 245 entered into final data analysis. Most samples (>90%) showed multiple chromosome abnormalities (median 8, range 1-64). The most frequently affected chromosome arms were: 1p (54%), 6p (39%), 6q (38%), 18q (36%), 12q or 16p (33%), and 1q (32%). Specific common CNAs were deletions of 1p, 6q, 10q, and 17p, gains of X, 1q, 2, 7, 8q, 12, 17q, and 18, and cnLOH of 1p, 6p, and 16p. Univariate analysis for progression free survival (PFS) identified several aberrations significantly associated with prognosis at unadjusted .05 level. Multivariate analysis adjusting for FLIPI risk, bulky disease, and combined serum b2M and LDH levels demonstrated that three markers, 9p deletion or cnLOH, 12p gain, and 17q gain, remained significant for PFS (HR 2.0, 1.7, and 1.7 for 9p, 12p, and 17q, respectively). Comparing treatment arms, the hazard of progression or death is 0.6 times less comparing CHOP+I-131 vs. CHOP+R if a patient has no abnormality in Xp (P=0.02). When we further analyzed specific segmental aberrations, eight regions were independent prognostic markers for overall survival (OS) in multivariate analysis and seven regions for PFS (see Figure 1 for representative markers). Specifically, patients with abnormal CDKN2A/B (p16) were 3.2 times more likely to progress early (P<.0001) while those with abnormal TP53 (17p) and TNFRSF12A/CREBBP (16p) loci were 3.9 and 6.7 times, respectively, more likely to die early (P=0.0001). For treatment effect on OS, six patients with abnormal TNFRSF12A/CREBBP (16p) showed an HR 0.06 when receiving CHOP+I-131 compared to CHOP+R (P=0.02). In particular, comparing patients who progressed vs. not at two years, Fisher's exact test identified seven differential genomic regions. Among these, FANCL region on 2p and USP3 region on 15q were also significant (P<.005) in Cox regression analysis limiting 2-year follow-up with the goal to focus on early events.
Conclusion: We confirmed the frequent 1p (TNFRSF14) and 6q (TNFAIP3) deletions in FL reported in literature. More importantly, we identified several genomic aberrations that showed independent prognostic value for FL patients. Among these, deletions and cnLOH of CDKN2A (9p), CREBBP (16p) and TP53 (17p) are of significant interest. These genomic aberrations may add to currently utilized clinical risk stratification as a means to identify high risk patients at diagnosis of FL. Certain markers that showed treatment arm effect may serve as predictive markers.
Support: This work was supported by Affymetrix Inc., NIH/NCI National Clinical Trials Network (NCTN) grants CA180888 and CA180819, and in part by GlaxoSmithKline.
Press: Roche: Honoraria, Research Funding; BMS: Honoraria; Bayer: Consultancy. Hsi: Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Cellerant Therapeutics: Research Funding; Abbvie: Research Funding; Eli Lilly and Co.: Research Funding. Leonard: Celgene: Consultancy, Research Funding; Roche: Consultancy. Friedberg: Bayer HealthCare Pharmaceuticals.: Other: Data and Safety Monitoring Board: Bayer HealthCare Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal